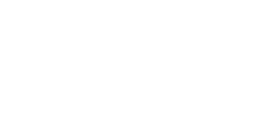By providing rapid and accurate diagnostic solutions, in the right place at the right time, we enable healthcare professionals, governments, individuals and organisations to make appropriate and informed decisions. We strive to address areas of high unmet need in infectious diseases.
We are constantly innovating to develop new products and improve existing solutions to provide the highest quality diagnostics that enable healthcare professionals to deliver optimal care.
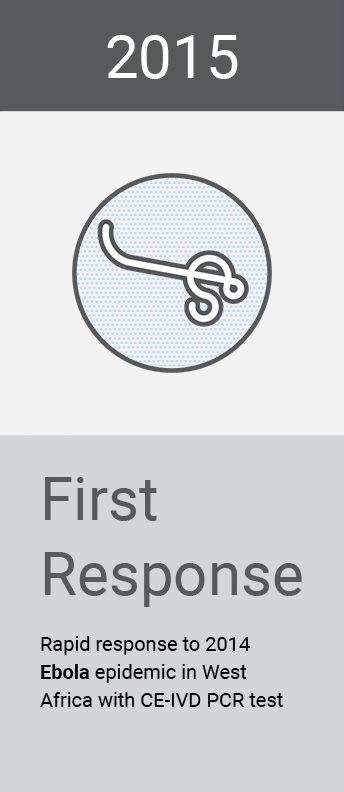
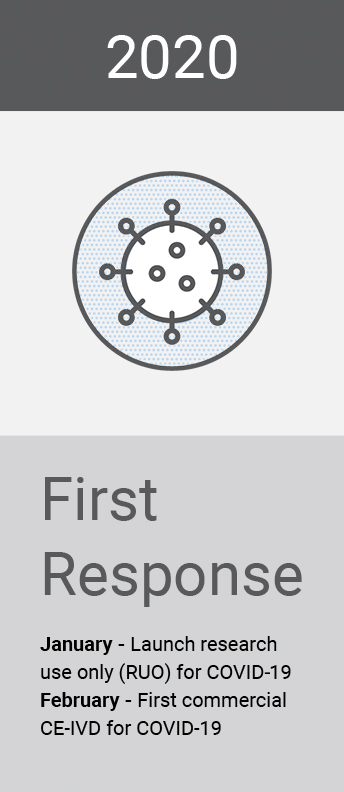
Our global surveillance expertise and relationships with the scientific community also help us to provide solutions to emerging or anticipated healthcare threats from around the world. Research and insights inform our activity and allow us to position ourselves as first responders in the global fight against infectious diseases.
We are a team of dedicated, passionate industry-leading experts, working from customers insights and evaluation to research and development, to manufacturing, to commercialisation.
We offer a comprehensive and developing portfolio of high-end products, backed by a global network of proven supply & delivery services. This ensures our end-to-end commitment to quality.
Supporting communities and wider society
At Novacyt, we believe in contributing to communities where we operate, and we have made donations to various charities and schools in the areas our offices are located. Following the transformational financial performance of Novacyt in 2020, a charity steering committee was created made up from a number of key employees within the group tasked with identifying schools and charities in need of support particularly following the COVID-19 pandemic. In 2021 a sum of £500,000 was dedicated to supporting a total of 25 schools and 50 charities throughout the year. We assisted projects looking to support critically ill children and adults, the homeless, old age pensioners and war veterans.
To support efforts to tackle the COVID-19 pandemic in Africa, Novacyt continues to work in partnership with non-governmental organisations to provide testing across the globe.
The Novacyt group is proud to play a part in supporting local communities and is truly humbled by the impact our charitable donations continue to make under the leadership of our charity steering committee on a yearly basis moving forwards.



Quality Policy
Within Primer Design Limited (part of Novacyt Group), we are committed to Quality as a fundamental part of our business activities and where possible exceed the requirements and expectations of our customers. We proactively seek opportunities for improvements in service and product quality whilst meeting all regulatory requirements for the markets we serve. Our aim is to safeguard people from the world’s infectious diseases by focusing on our customers’ needs in a proactive, responsive and honest manner.
- Quality for our company – to involve all employees in owning and improving the Quality Management System to the benefit of customers, employees and other interested parties. To place Quality as a fundamental cornerstone of annual business strategic planning
- Quality for our customers – to monitor and measure the performance of the products we sell, and the systems and processes we operate, ensuring we identify and implement continuous improvements
- Quality for our employees – to drive a Quality culture by emphasising the importance of Quality through regular communication, ensuring that employees are trained and supported to deliver high quality products and services
- Quality for regulatory authorities – to maintain a Quality Management System that complies with the requirements laid down in the IVD Directive (98/79/EC), IVD Regulation (2017/746), ISO 9001, ISO 13485 and other regulations as required to support business activities
This Quality Policy will be reviewed annually for continuing suitability.